- RESOURCE CENTER
Scientific Resources on Stem Cell Therapies
Explore Our Regenerative Medicine library

F.A.Q.

What Are Stem Cells?
Types of Stem Cells

Source of Our Stem Cells
Conditions Treated
Third-Party Publications
Is This Treatment Available in the U.S.?
Patient Testimonials
- CONDITIONS
Conditions treated with Regenerative Medicine
Regenerative medicine plays a critical role in modulating inflammation and immune responses, offering therapeutic potential for a variety of health conditions.
- PROTOCOLS
Our Signature Protocols
Tailored stem cell therapies, meticulously designed to target specific conditions.
- WELLNESS RETREATS
- TESTIMONIALS
- ABOUT
Multiple sclerosis stem cell treatment breakthrough could stop disease in its tracks
Multiple sclerosis stem cell treatment breakthrough could stop disease in its tracks
DISCOVER REGENERATIVE MEDICINE
U-MSC stem cell treatments offer a promising path to improving the quality of life for patients with multiple sclerosis.
A clinical study found injecting stem cells into the brains of MS patients could potentially stop the disease from advancing.
In a significant advancement in stem cell research, a clinical study has shown promising results in halting the progression of multiple sclerosis (MS). This breakthrough, detailed in an article by Rebecca Thomas and Jabed Ahmed for The Independent in November 2023, offers a new potential treatment avenue for MS, a debilitating neurological condition.
Study Findings and Methodology
The study, conducted by researchers from the University of Cambridge and other international teams, involved injecting stem cells into the brains of 15 patients with secondary progressive MS. These stem cells, derived from the brain tissue of a miscarried fetal donor, showed promising results in stopping the disease’s advancement. The Independent reported that The procedure was “safe, well tolerated, and had a long-lasting effect that appears to protect the brain from further damage.”
Patient Experiences and Perspectives
Lisa Haines, a 55-year-old MS patient, shared with The Independent her positive experience with a similar stem cell treatment in Mexico, noting significant improvements in her symptoms. Her story underscores the potential benefits of stem cell therapy for MS patients, particularly for those who have not found relief through conventional treatments.
Research Outcomes and Potential Implications
Throughout the 12-month follow-up period, no treatment-related deaths or serious adverse effects were observed. The study, published in the journal Cell Stem Cell, reported a “substantial stability of the disease, without signs of progression.” However, researchers acknowledged the limitations of the small study size and the potential confounding effects of immunosuppressant drugs.
Expert Opinions and Future Directions
Caitlin Astbury, research communications manager at the MS Society, described the study as “exciting,” adding that further clinical trials are necessary. Dr. Aravinthan Varatharaj, a clinical lecturer in neurology at the University of Southampton, emphasized the need for more evidence on the technique’s ability to repair or regrow nerve cells.
Professor Stefano Pluchino, co-leader of the study from the University of Cambridge, expressed cautious optimism about the findings, highlighting the study’s implications for developing new treatments for secondary progressive MS.
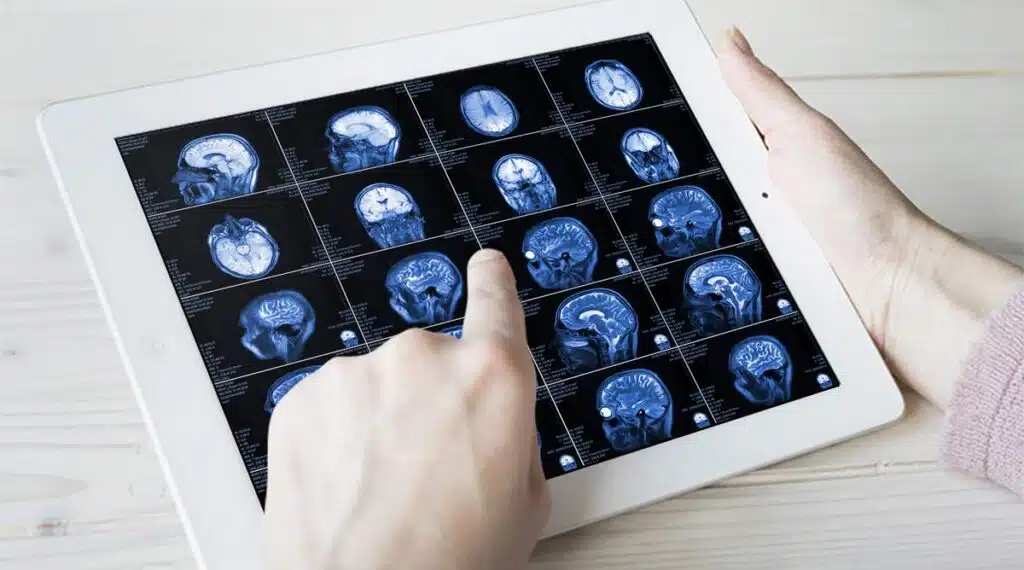
As reported by The Independent, this breakthrough in stem cell treatment for MS marks a significant step in the fight against this challenging disease. While acknowledging the need for further research and more extensive clinical trials, these early findings offer hope for those affected by MS and point towards the potential of stem cell therapy in changing the course of this disease.
This article has been adapted for publication on the Omni-Stem website to inform and educate about the latest developments in stem cell research for multiple sclerosis treatment, based on the original report by Rebecca Thomas and Jabed Ahmed in The Independent. Read Original Article

To access more information, visit our Resource Center. There, you’ll find a comprehensive collection of resources, studies, and recent advancements in Regenerative Medicine
Stem cell treatments are currently deemed experimental and have not yet received FDA approval. As a result, such treatments are not permitted in the U.S. While this doesn’t imply that these treatments are ineffective, it does indicate that there’s limited scientific evidence supporting their use.

To access more scientific papers, visit our Resource Center
Stem cell treatments are currently deemed experimental and have not yet received FDA approval. As a result, such treatments are not permitted in the U.S. While this doesn’t imply that these treatments are ineffective, it does indicate that there’s limited scientific evidence supporting their use.
DISCOVER REGENERATIVE MEDICINE
U-MSC stem cell treatments offer a promising path to improving the quality of life for patients with multiple sclerosis.